blog
Read our insights on what’s happening in the industry today.

Providing the Visibility Needed to Achieve Representation in Clinical Research
Fully featured CTMS and EDC can provide the visibility into enrollment trends needed to achieve representation in clinical research. Read how we use Oracle Clinical One to view and monitor participant sociodemographics and more.

Equitable Access to Research Is Intrinsic to Health Equity
Equity in research plays a vital role in equity in health and health care, starting with clinical trials and carrying through post-marketing access to new vaccines and treatments. Read this blog post for a deeper dive into where the industry stands with equitable access and how it contributes to the success of a trial.
Increasing Regulatory Harmonization in Africa is Streamlining Marketing Authorization of Your Products
Dissonance in regulatory processes and timelines between countries in Africa has been cited as a major deterrent to pursuing research in the area, but times are changing. Initiatives are successfully increasing regulatory harmonization and streamlining marketing authorization across the continent.

High-Quality Data and Growing Capacity Make Sub-Saharan Africa a Research Destination
With experienced researchers and sites, investment in research infrastructure by local and overseas organizations as well as engaged stakeholders, Africa should be a research destination for pharma, biotech, government and non-governmental organizations alike.

Developing Treatments Against COVID-19: a Focus on Africa
One strategy to reaching endemic status for COVID-19 globally is equitable access to vaccines and therapies. Studies such as the one we’re supporting in Ghana will help with this.
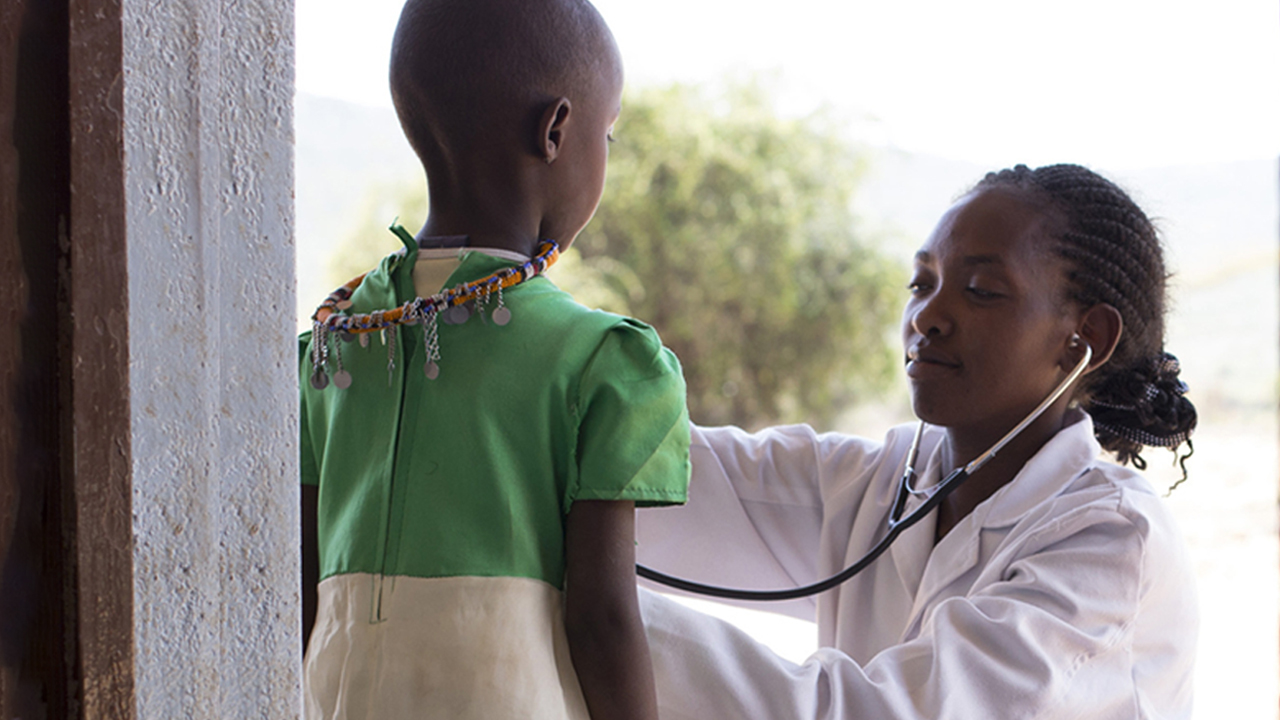
Increasing Clinical Research In Africa Presents An Opportunity To Address Global Health Challenges
Although the diversity of Africa is often cited, the full extent is still not well understood. As we learn more, the true potential of the continent for clinical research to address ongoing public health issues becomes apparent.
Acknowledging the Dynamic Epidemiology of Infectious Diseases During Clinical Trial Planning
The dynamic nature of infectious disease epidemiology requires diligent surveillance to ensure clinical trials of vaccines and treatments can successfully recruit and account for changing seroprevalence and new variants.

Q&A From Our Webinar — Africa’s Potential for Clinical Research: Population Diversity, Expanding Therapeutic Area Expertise and Long-Standing Stakeholder Engagement
In our recent webinar, our panel of esteemed speakers broke stereotypes about Africa, discussed research-related trends and highlighted how clinical trials could benefit from the continent’s strengths.

