Ensuring health equity has long been key to the mission of FHI 360, our parent company. One reason that FHI 360 formed a specific clinical trials subsidiary, FHI Clinical, was the recognition that equity in research plays a vital role in equity in health and health care, and that has underpinned our mission since we formed FHI Clinical in 2019.
Equity in research is essential for a number of reasons. First, because the efficacy of therapies and vaccines depend on many intrinsic and extrinsic factors, the clinical trial population must reflect the characteristics of the product’s end user in real life: sex, gender, race, ethnicity, age, ancestry, social determinants of health, environmental factors, genetics, comorbidities, concurrent medications, education, profession and more. Second, the research community needs to ensure equitable protection from drug-related risks. Without the inclusion of all people who will ultimately use the drug, research fails to document the safety risks and thresholds for all.
Adequate representation in clinical trials can also challenge systemic barriers in access to treatment and care in general, particularly as participants gain knowledge about their disease, the investigational product and/or other other health resources that could benefit them and their communities. Let’s dive a little deeper into the significance of equitable access to research.
Equity vs diversity
Equity is ensuring that everyone has a fair and equal chance of participating, while diversity is the inclusion of people of different genders, ages, races, cultures, socioeconomic statuses, education levels, etc in the clinical trial.
One aim of equitable access to clinical trials is making sure trials are being conducted in the populations that would benefit from them and who have the disease characteristics of interest.
Trials with diverse populations can help to characterize the disease and treatment/vaccine effects and safety in as broad a range of people as possible.
Both concepts contribute to representativeness in a clinical trial — that the clinical trial population resembles the real-life patient population as closely as possible. However, equitable access does not always mean a heterogeneous population, and having a diverse population doesn’t guarantee that the most at-risk populations were offered the opportunity to participate.
Disparity persists in research access
Although COVID-19 highlighted the racial and socioeconomic disparities in access to vaccine and therapeutic research, especially in the United States,1 the issue is not new. Moreover, many populations globally are under-represented in clinical trials, as we recently reported in a blog post about research in Africa. Studies have reported under-representation in clinical trials across the participation continuum: knowledge and understanding of trials, intention to participate, being approached by the research team, the effort needed to enroll and actually successfully enrolling in the trial.2,3
Racial/ethnic minority groups in the United States remain the most affected by HIV/AIDs. In a study of preventive HIV vaccine clinical trials in the United States, enrollment of these racial/ethnic minority groups increased 94% between 2002 and 2016.4 However, despite this increase, of all the participants, only 32.7% identified themselves as a racial/ethnic minority, meaning racial/ethnic minorities were still under-represented in clinical trials. Moreover, 1.9 to 2.9 times more Black people were diagnosed with HIV than participated in HIV clinical trials during the same period, and 1.3 to 6.6 times more Hispanic/Latino people were diagnosed with HIV than participated in HIV clinical trials.
Racial/ethnic minority groups remain under-represented in clinical trials
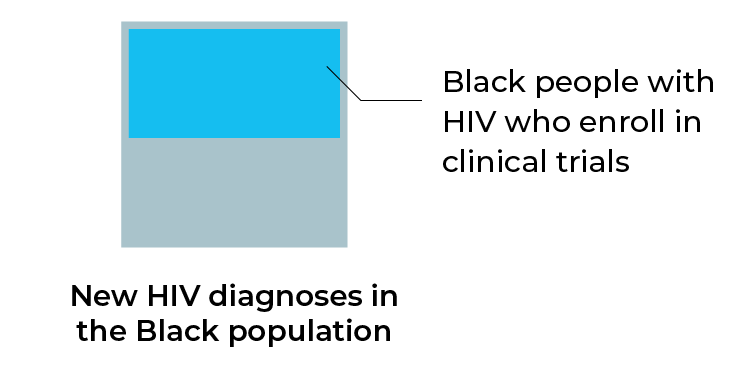
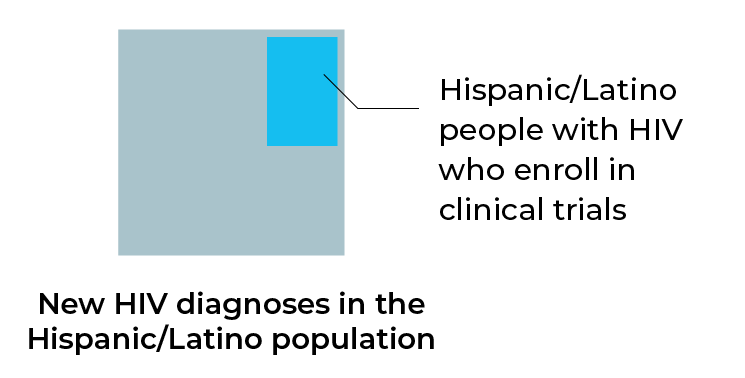
Similarly, although enrollment of racial/ethnic minority groups into diabetes treatment trials has increased, it is not proportional to the distribution of the non-White diabetes population.4,5 Interestingly, although increased non-White ethnic enrollment was observed globally in one review, this was not the case in the United States and United Kingdom. Under-enrollment of non-White ethnic groups in the United States was 62.3% of the same population who have diabetes.5
Similar under-representation of non-White groups has been reported for clinical trials for heart failure,6 systemic lupus erythematosus,7 diabetes,5 Alzheimer’s disease8 and dementia,9 despite higher disease risk and prevalences and poorer outcomes for these diseases in non-White populations.
This has particular importance because studies have found race-specific responses to HIV preventive vaccines,10,11 and participants with predominantly Asian ethnicity have different responses to diabetes treatments than White participants.12 Other studies have found a protective effect of mutations in an LDL cholesterol pathway that are present in individuals of African descent with low LDL cholesterol levels but not in those of European descent with low LDL cholesterol levels,13 as well as higher prevalence of rheumatic heart disease (RHD) in Black Africans than in other populations.14
We’re failing to reach and educate the populations most at risk. For example, in a survey of HIV-infected adults receiving HIV care at all 47 domestic AIDS Clinical Trials Group clinical research sites in the United States, the South had the lowest proportion of respondents who were aware of HIV studies or had ever had studies discussed with them, despite the highest rates of new HIV diagnoses in the country.2 Moreover, African American respondents living in the South were most likely not to participate in research because they did not understand it.
Delivering trials to those most affected: a comprehensive approach
Many national and international initiatives have been formed to encourage the inclusion of more participant groups15; however, these general recommendations are challenging to implement at an individual trial level. Successful inclusion of representative populations needs to be approached during protocol design for each trial — a one-size-fits-all approach is not effective. Instead, researchers should focus on getting trials to the population hardest hit by the condition or disease.
Based on our experience with global trials, the FHI Clinical approach includes the following:
- Characterize the population with the disease or condition of interest
- Evaluate where people with those characteristics are located
- Identify competent sites within those areas
- Apply cultural competence to recruitment and retention strategies, including through dedicated Social Mobilization and Communications departments, such as we have for PREVAIL
Case study: using epidemiological and biosurveillance methods to guide site selection in tuberculosis vaccine trials
One strategy for equitable access to clinical trials is to use real-world data to determine the actual disease and population characteristics, followed by finding people who match those characteristics, regardless of gender, age, race, socioeconomic status, etc. The FHI Clinical Global Strategy team uses this approach to achieve their goal of promoting clinical equity through research and discourse on trial design at the global level.
An example of this in practice is the team’s feasibility assessment for a potential Phase 3 clinical trial of a tuberculosis (TB) vaccine, in collaboration with internal and external partners. A core challenge was finding high-incidence TB hotspots that also have investigators at sites capable of conducting the trial: Do they have the right equipment? Can we move things in and out? Can we ship? Further complicating the situation was the wide variation in TB preventive treatment (TPT) policies and implementation, which influences eligibility for a vaccine trial.
Our aim was to rapidly and systematically identify about 50 sites globally with both a high TB burden and the capacity to conduct a Phase 3 clinical trial, rapidly and in a systematic way. The traditional site feasibility process tends to be a linear process and often relies on the familiarity with a particular site by the CRO, sponsor, key opinion leader or stakeholder(s). As a result, these studies often have limited potential reach to the highest burden populations, and we’re overlooking potential populations that might be eligible for inclusion in a clinical trial.
To be more equitable in our site selection, we identified five key criteria to evaluate potential sites. These criteria were weighted to prioritize TB incidence (to ensure the vaccine efficacy endpoint would be reached) and site capacity (ability to manage an active TB trial such as access to a lab to collect and ship samples), followed by accessibility, experience and regulatory landscape.
To gather the data needed to evaluate these criteria, we integrated biosurveillance methodology and systematic epidemiological evaluation into the traditional site feasibility process. Expert epidemiological analysis of the incoming data also allowed us to identify any anomalies that needed to be clarified by the sites. This combination of methods instilled high confidence in the final scoring of each site and allowed us to identify specific catchment areas using quantifiable indicators.
Using this systematic, objective evaluation also allowed us to shorten the evaluation period and operationalize equity and accessibility to trials.
For more insights and to hear audience questions, check out the on-demand recording of the plenary session discussion on this topic.
Expanding beyond typical site locations to reach additional populations affected by a disease can have a significant impact on the characteristics of the study sample.8 Further, consideration should be given to limiting exclusion criteria to only those that are necessary. The use of very restrictive exclusion criteria not only narrows the eligible population but also fails to reflect the real-world situation in which the product will be used.8,16
Culturally sensitive approaches to engaging the populations of interest is paramount to overcome barriers to participation in clinical trials. For example, reasons for not participating in a study conducted in the United States included negative family and community opinions about research, mistrust from the African American community and language barriers for Hispanic immigrants.3
As we reported in our blog post about cultural competence, the following should be considered during the study design phase:
- Incorporation of input from your study population about recruitment, consent and other study-related information and procedures, including study design, when appropriate
- How participants’ perspectives might influence their willingness to consider, enroll or stay in a research study
- The political, social and individual contexts that could influence understanding of the research and the informed consent process
- How to accomplish bi-directional information exchange and what information (and in what formats) is relevant to the target population
- Whether existing data collection tools are valid and appropriate for the setting and population
- If the interventions should be adapted to align with cultural beliefs, values and practices
- The inclusion of sites and researchers who also reflect the population of interest, who might be better-positioned to engage participants
Applying this to trial design requires more than translation of documents to additional languages. It can include forming a community advisory board; employing community engagement staff and community educators; and incorporating feedback from scientists, researchers and health care providers from the populations of interest. Feedback from these groups can help determine if incentives for participation need to be provided to address social determinants of health; implementation of mobile units and satellite clinics would improve access; and material could be improved by ensuring respectful language, inclusive identifiers and clearer structure.17
Researchers involved in the COVID-19 Prevention Network (CoVPN) reported that relationships they established with Tribal nations helped them respect tribal sovereignty, by collaboratively developing contracts that outlined sharing and ownership agreements for tribal data, material and biospecimens. The result was greater participation by people from those nations.18 Such strategies of engagement with local experts to understand community hierarchy and expectations have also been important in FHI Clinical’s research in Latin America, Asia and Africa.
Partnerships with trusted community leaders — such as advocacy organizations, health care providers, faith-based organizations and media — can also be instrumental in opening communication channels and disseminating information about the intended benefit, safety and side effects of investigational products. They can also act as important referral channels.
In addition, it is not sufficiently effective to implement culturally sensitive strategies at a study level — individual researchers supporting the study should also practice cultural competence when interacting with participants of different cultures and backgrounds. Investing in cultural awareness training for staff could enhance recruitment and retention, as demonstrated within the HIV Vaccine Trials Network (HVTN).4
Additional considerations to ensure broad, equitable uptake of the intervention in the post-marketing period include an understanding of the target population’s needs and desires from a new product — to help define study questions and outcomes that are important to the end users. The community affected by HIV has long advocated for research involvement so they can better understand, prevent and care for the disease and have gathered valuable input from people living with HIV, sex workers, people who inject drugs, transgender people and men who have sex with men.18 Their input into trial design has helped ensure vaccine and treatment efficacy, not only from a biological sense but also from the perspective of acceptability of testing, prevention and treatment delivery mechanisms (devices and interactions with healthcare providers).
Broad input during trial planning key to equitable research access
All researchers have a role in ensuring populations have access to the care they need, starting with clinical trials and carrying through post-marketing access to new vaccines and treatments. This could be of particular importance for commercial sponsors to consider; a study found that industry-funded studies had significantly increased odds of under-enrollment of all non-White groups when compared with funding from a public body or institute.5
Stepping beyond the four walls of the office or lab to connect with the community and gather important epidemiological information during the trial planning phase contributes not only to more representativeness of the study population but also to the overall success of the trial.
Contact our team to learn more about our approach to site selection and participant recruitment and retention.
References
- Mackey K, Ayers CK, Kondo KK, et al. Racial and ethnic disparities in COVID-19-related infections, hospitalizations, and deaths: a systematic review. Annals of Internal Medicine 2021;174(3):362–373. doi: 10.7326/m20-6306.
- Heumann C, Cohn SE, Krishnan S, et al. Regional variation in HIV clinical trials participation in the United States. South Med J 2015;108:107-116. https://doi.org/10.14423/smj.0000000000000234
- Castillo-Mancilla JR, Cohn SE, Krishnan S, et al. Minorities remain underrepresented in HIV/AIDS research despite access to clinical trials. HIV Clin Trials 2014;15:14-26. https://doi.org/10.1310/hct1501-14
- Huamani KF, Metch B, Broder G, et al. A demographic analysis of racial/ethnic minority enrollment into HVTN preventive early phase HIV vaccine clinical trials conducted in the United States, 2002–2016. Public Health Rep 2019;134:72-80. https://doi.org/10.1177/0033354918814260
- Li G, Zhang J, Van Spall HGC, Douglas PS, Wang Y, Sun X, Thabane L. Exploring ethnic representativeness in diabetes clinical trial enrolment from 2000 to 2020: a chronological survey. Diabetologia. 2022 Jun 16:1–12. doi: 10.1007/s00125-022-05736-z
- Wei S, Le NC, Zhu JW, et al. Factors Associated With Racial and Ethnic Diversity Among Heart Failure Trial Participants: A Systematic Bibliometric Review. Circulation: Heart Failure 2022;15(3):e008685
- Williams JN, Dall’Era M, Lim SS, et al. Increasing Ancestral Diversity in Systemic Lupus Erythematosus Clinical Studies. Arthritis Care Res 2022;74:420-426. https://doi.org/10.1002/acr.24474
- Franzen, S, Smith, JE, van den Berg, E, et al. Diversity in Alzheimer’s disease drug trials: The importance of eligibility criteria. Alzheimer’s Dement 2022;18:810-823. https://doi.org/10.1002/alz.12433
- Bothongo P, Jitlal M, Parry E, et al. Dementia risk in a diverse population: a single-region nested case-control study in the East End of London. Lancet Reg Health Eur 2022;15:100321.
- Gilbert PB, Peterson ML, Follmann D, et al. Correlation between immunologic responses to a recombinant glycoprotein 120 vaccine and incidence of HIV-1 infection in a phase 3 HIV-1 preventive vaccine trial. J Infect Dis 2005;191(5):666-677.
- Montefiore DC, Metch B, McElrath MJ, et al. Demographic factors that influence the neutralizing antibody response in recipients of recombinant HIV-1 gp120 vaccines. J Infect Dis 2004;190(11):1962-1999.
- Gan S, Dawed AY, Donnelly LA, et al. Efficacy of modern diabetes treatments DPP-4i, SGLT-2i, and GLP-1RA in White and Asian patients with diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Care. 2020;43(8):1948–1957. doi: 10.2337/dc19-2419.
- Cohen J, Pertsemlidis A, Kotowski IK, et al. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat Genet 2005;37:161-165. https://doi.org/10.1038/ng1509
- Machipisa T, Chong M, Muhamed B, et al. Association of Novel Locus With Rheumatic Heart Disease in Black African Individuals: Findings From the RHDGen Study. JAMA Cardiol 2021;6(9):1000-1011. doi:10.1001/jamacardio.2021.1627
- Enhancing the Diversity of Clinical Trial Populations — Eligibility Criteria, Enrollment Practices, and Trial Designs Guidance for Industry. Food & Drug Administration. November 2020. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/enhancing-diversity-clinical-trial-populations-eligibility-criteria-enrollment-practices-and-trial
- Schneider LS, Olin JT, Lyness SA, et al. Eligibility of Alzheimer’s disease clinic patients for clinical trials. J Am Geriatr Soc 1997;45(8):923-928.
- Andrasik MP, Broder GB, Wallace SE, et al. Increasing Black, Indigenous and People of Color participation in clinical trials through community engagement and recruitment goal establishment. PLoS ONE 2021;16(10):e0258858. https://doi.org/10.1371/journal.pone.0258858
- Pantelic M, Steinert JI, Ayala G. Addressing epistemic injustice in HIV research: a call for reporting guidelines on meaningful community engagement. J Int AIDS Soc 2022;25:e25880. https://doi.org/10.1002/jia2.25880

