Only ~20% of the attendees in our recent webinar, Africa’s Potential for Clinical Research, had been involved with research conducted in Africa, and an additional 40% had conducted no more than five studies. At FHI Clinical, we see this as a missed opportunity for many organizations developing medicines and vaccines to take advantage of experienced, knowledgeable sites and investigators and to access unique populations, many of whom would benefit from investigational interventions.
How much experience do you have conducting research in Africa?
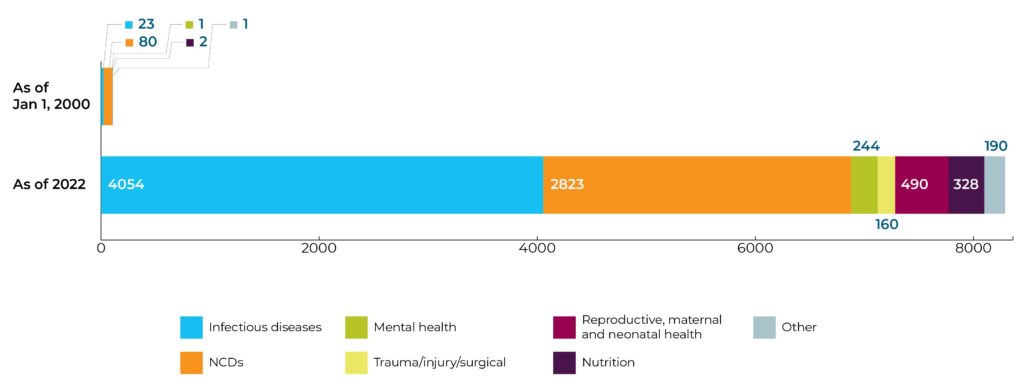
Research portfolio is flourishing
- South Africa
- Nigeria
- Kenya
- Uganda
- Tanzania
- Malawi
- Ethiopia
- Ghana
- Zambia
- Zimbabwe
Research capacity is growing with local investment
- Strengthen capacity for sustained, integrated, coordinated and collaborative research, innovation and translation for health
- Develop and implement sustainable mechanisms for investment and financing in research and innovation for health
- Generate new knowledge aligned to health goals and targets and advocate for its translation into products, services, policies and practices to improve health
- Strengthen data-sharing platforms and systems to optimize health delivery
- Advocate for the adoption of emerging technologies and supporting platforms to improve health
- Strengthen and harmonize regulatory, ethics and intellectual property systems in order to maximize the benefits from African-led research and innovation for Africa and the global community
- Maximize the benefits from African-led research and innovation for Africa and the global community through robust research regulatory and intellectual property systems
Mentorship of new researchers has also played an important role in developing early-career investigators. In our webinar, Dr. Mookho Malahleha, CEO of Synergy Biomed Research Institute in South Africa, described her personal experience with mentorship as a young researcher.

Proven, resilient sites and investigators contribute to robust research
- WHO-GMP
- Malaria in Pregnancy consortium
- International and national regulatory agencies
- University of the Witwatersrand in Johannesburg
- Government departments
- Ministries of health
- Ethics committees
- Local communities
- Current WHO-Global Malaria Program (GMP) malaria treatment guidelines
- Different strains in different countries, including treatment-resistant strains
- Different regulatory and ethics approval processes
- Verified treatment compliance
- Sought participants who missed visits
- Performed postnatal home visits
Capitalize on research opportunities in SSA
References
- Edem B, Onwuchekwa C, Wariri O, et al. Trends in clinical trial registration in sub-Saharan Africa between 2010 and 2020: a cross-sectional review of three clinical trial registries. Trials 2012;22:472. https://doi.org/10.1186/s13063-021-05423-1
- Health Research and Innovation Strategy for Africa (HRISA): 2018-2030. African Union Development Agency (AUDA-NEPAD). Available at: https://www.nepad.org/publication/health-research-and-innovation-strategy-africa-hrisa-2018-2030. Accessed on April 15, 2022.
- Kasprowicz VO, Chopera D, Waddilove KD, et al. African-led health research and capacity building- is it working? BMC Public Health 2020;20:1104. https://doi.org/10.1186/s12889-020-08875-3
- Mtove G, Kimani J, Kisinza W, et al. Multiple-level stakeholder engagement in malaria clinical trials: addressing the challenges of conducting clinical research in resource-limited settings. Trials 2018;19:190. https://doi.org/10.1186/s13063-018-2563-1

