Our Experience in sub-Saharan Africa
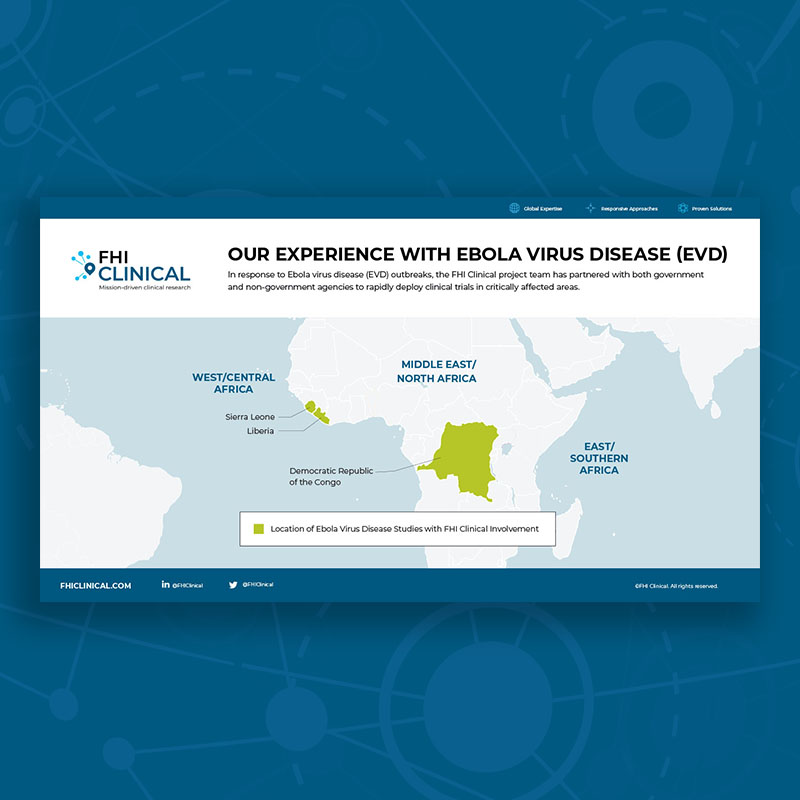
FHI Clinical has long-standing relationships, a local team and broad expertise in sub-Saharan Africa. We're ready to help you confidently expand your research across the region, finding the best-fit countries and populations for your target indication and study type.