mRNA Report
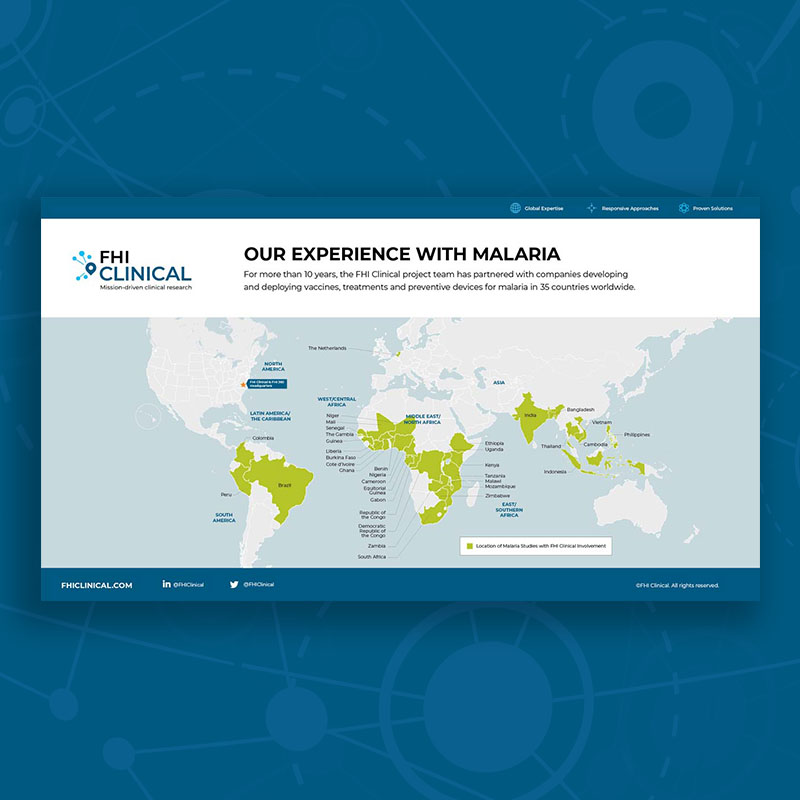
mRNA technology has the potential to address diseases globally that have not had effective vaccines nor treatments to date. Read this report to learn about the promise of mRNA to prevent and treat endemic diseases in resource-limited areas with the populations who would benefit the most.