BUILDING AND SUSTAINING RESEARCH CAPACITY
BUILDING RESEARCH CAPACITY
Watch the video to learn how our teams support sites to develop sustainable research capacity and produce high-quality study data. In addition, we work with regional institutions to establish networks of research-ready sites that focus on local health and research priorities.


Partnership of Clinical Research in Guinea (PREGUI) and Partnership for Research on Vaccines and Infectious Diseases in Liberia (PREVAIL)

China Tuberculosis Clinical Trials Consortium (CTCTC)
In September 2013, Beijing Chest Hospital (BCH) initiated the China TB Clinical Trials Consortium (CTCTC) as a multi-site, national consortium dedicated to building research infrastructure for multinational TB clinical trial research collaboration in China.
From 2013 through 2020, FHI Clinical provided technical support and guidance to implement strategies for clinical trial capacity building as well as online, on-site or workshop-based training to develop research capacity.
SUSTAINING RESEARCH CAPACITY
Keeping a site functional preserves investment in human and equipment resources and ensures the site is ready to conduct quality research as soon as there is a need.
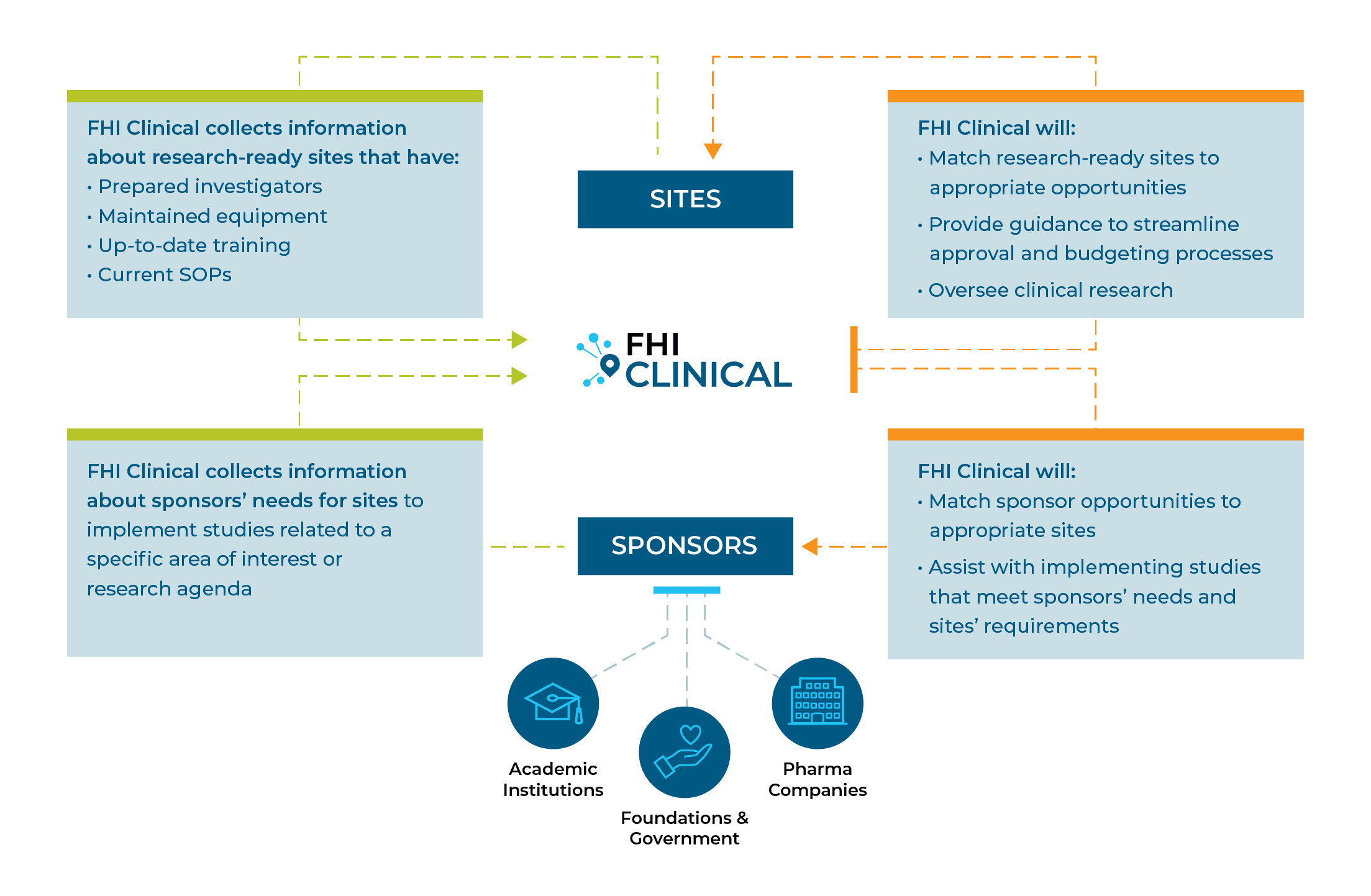
Working together, we can develop high-quality sites that are ready to accept new projects.





