Background
Study Duration: 2013–2020
FHI Clinical Funding: U.S. National Institutes of Health (NIH) National Institute of Allergy and Infectious Diseases (NIAID)
Therapeutic Area: Infectious Diseases
Initiative: China TB Clinical Trials Consortium (CTCTC)
Initiative Funding: U.S. NIH, NIAID, Government of China
Tuberculosis (TB) remains one of the top 10 causes of death worldwide, surpassing both HIV/AIDS and malaria, and it is the leading cause of death from a single infectious agent.1 Because it poses a greater risk for immunocompromised individuals, TB is a leading killer of HIV-positive people.1 Further, multidrug-resistant TB (MDR-TB), caused by bacteria that do not respond to the two most effective first-line anti-TB drugs, is considered a serious public health issue.
Although TB occurs worldwide, eight countries accounted for two-thirds of the new TB cases in 2017 (in descending order): India, China, Indonesia, the Philippines, Pakistan, Nigeria, Bangladesh and South Africa. Similarly, low- and middle-income countries account for more than 95% of TB deaths, primarily in seven countries (in descending order): India, Indonesia, China, Philippines, Pakistan, Nigeria and South Africa. And, almost half of the global drug-resistant cases occurred in three countries (in descending order): India, China and the Russian Federation.
TB is both preventable and curable — between 2000 and 2017, an estimated 54 million lives were saved through TB diagnosis and treatment.2 Yet, the global TB incidence is decreasing only 2% annually.
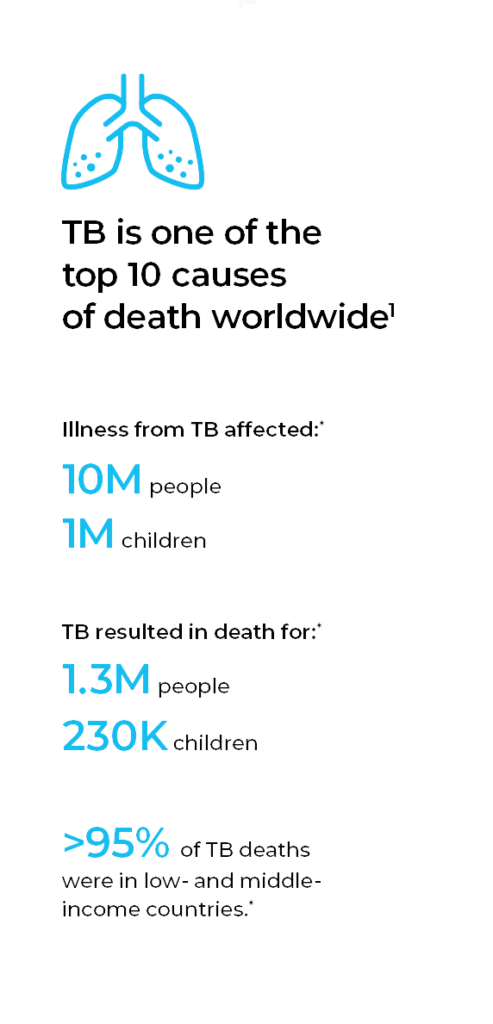
Challenge
TB elimination remains challenging for many reasons. An effective vaccine to prevent TB in adults has yet to be developed. Not everyone who is exposed or infected with TB-causing bacteria will become ill, and it is difficult to predict who will become ill, as there are few informative biomarkers or correlates of immunity. Accurate, timely diagnosis, particularly of MDR-TB or HIV-associated TB disease, can be complex and expensive, requiring sophisticated devices and systems that are not available in some settings. After diagnosis, lengthy treatment requirements can decrease adherence and create risk of disease re-occurrence. A standard six-to-nine-month course of four antimicrobial drugs (established 40+ years ago) is used to treat active, drug-susceptible TB, and treatment toxicities can occur. Control of MDR-TB is the current biggest treatment challenge — with only a 55% global treatment success rate.1
Although research is needed to advance TB diagnosis and treatment, capacity for clinical research tends to be limited in high TB burden settings.
Challenges for TB diagnosis
and treatment:
- No effective preventive vaccine
- Inability to predict who
develops TB - Few informative biomarkers
- Sophisticated diagnostic equipment
- Lengthy treatment
- Treatment toxicities
- Limited research capacity in high TB burden settings
Solution
With its large population, extensive hospital network, high TB burden, and low HIV rate, China provides a unique patient pool for TB clinical studies — one that has not yet been fully utilized. In September 2013, Beijing Chest Hospital (BCH), which also serves as the Clinical Center of TB (CCTB), China Center for Disease Control (CDC), initiated the China TB Clinical Trials Consortium (CTCTC). It is a multi-site, national consortium dedicated to building research infrastructure for multinational TB clinical trial research collaboration in China — to develop novel anti-TB agents, new pre-exposure vaccines, and diagnostics.
We provided technical support and guidance to implement strategies for clinical trial capacity building. In addition, we partnered with other organizations to participate in the CTCTC, including the University of North Carolina Institute for Global Health & Infectious Diseases.
Capacity-building online, on-site or workshop training provided by the FHI Clinical project team:
Scientific knowledge regarding TB treatment
and monitoring
Study design
and clinical
trial development
International Conference on Harmonisation (ICH) Good Clinical Practice (GCP)
Research ethics and Institutional Review Board (IRB) operations/registration
Good Clinical Laboratory Practice (GCLP) and laboratory procedures for TB
Data management in
clinical research
Quality management, including risk-based monitoring and quality assurance & quality control (QA & QC)
Pharmacy management
Results
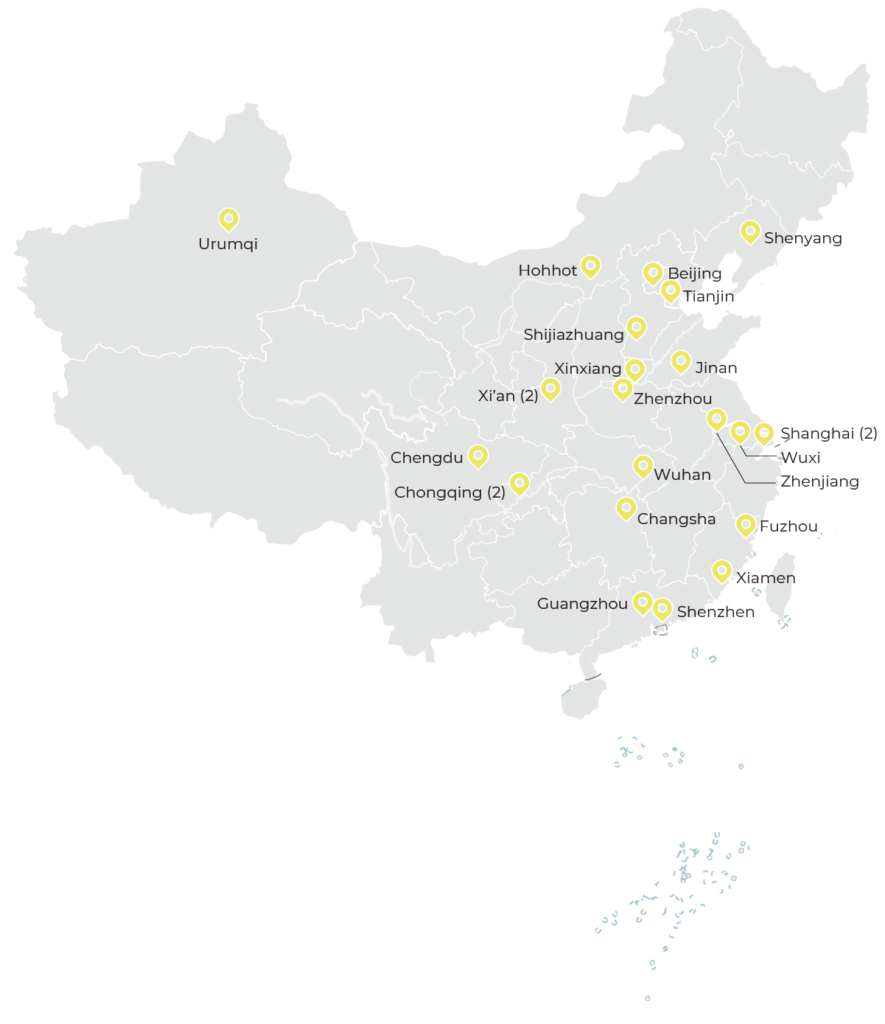
As of the end of 2020, the CTCTC hospital network consisted of 24 TB clinical and research institutions (22 certified by the National Medical Products Administration), funded by the Government of China. The CTCTC has established monitoring procedures to assure internationally recognized levels of TB laboratory research capacity, site staff training, and relationships with the international TB scientific and pharmaceutical communities. For junior investigators, the program has provided training, mentoring and small grants for TB-related research projects.

as of the end of 2020, The CTCTC hospital network consisted of 24 TB clinical and research institutions in China.
Standardized Treatment Regimen of Anti-tuberculosis Drugs for Patients with MDR-TB (STREAM)
The STREAM project presented an opportunity to leverage the CTCTC in China for TB research:
- Multi-national trial of bedaquiline treatment for MDR-TB patients
- FHI Clinical project team awarded the preparatory work for China: implementation and site support — training, mentoring and import/export logistics
- Four CTCTC hospitals were selected and trained
FHI 360, FHI Clinical’s parent company, has also been involved in the following TB studies in China:
International Maternal Pediatric Adolescent AIDS Clinical Trial (IMPAACT) Network
P1108: Phase I/II trial of bedaquiline treatment for HIV-infected and
HIV-uninfected pediatric populations with MDR-TB in Haiti, India and
South Africa
P1078: Phase IV trial to evaluate the safety of immediate vs deferred isoniazid preventive therapy for HIV-infected pregnant women and the infants born to the HIV-infected mothers in Botswana, Haiti, India, South Africa, Tanzania, Thailand, Uganda and Zimbabwe
2001: Phase I/II trial of once-weekly rifapentine and isoniazid for HIV-1-infected and HIV-1-uninfected pregnant and postpartum women with latent TB infection in Haiti, Kenya, Malawi, Thailand and Zimbabwe
2005: Phase I/II trial of delamanid in combination with an optimized background regimen (OBR) for HIV-infected and HIV-uninfected pediatric populations with MDR-TB in Botswana, India, South Africa and Tanzania
Located at FHI 360, the IMPAACT Operations Center is responsible for supporting the development, implementation, and reporting of all IMPAACT scientific protocols and for providing a central point of coordination, communications, and support to the IMPAACT Leadership Group and all network committees, protocol teams, and working groups. The Operations Center is also responsible for arranging and supporting all network meetings and leadership travel; for the governance of the network and communications; and for providing logistical and administrative support for the IMPAACT leadership and other committees.
Staff from the Operations Center work closely with the IMPAACT leadership, protocol teams, staff from the Statistical and Data Management Center (SDMC), Laboratory Center (LC), CTUs, Division of AIDS (DAIDS), the network committees and CTU community programs on all aspects of the IMPAACT research agenda.
IMPAACT Operations Center responsibilities include:
- Leadership and governance support
- Research management and support
- Protocol development and review
- Protocol-specific training of sites
- Oversight of clinical trial units during study conduct
- Coordination and facilitation of network committees
- Communication and information dissemination
resources
1. World Health Organization. Global Tuberculosis Report 2018. Geneva: World Health Organization. 2018.
2. World Health Organization. Tuberculosis fact sheet. Sept 18, 2018. Available at https://www.who.int/news-room/fact-sheets/detail/tuberculosis. Last accessed on July 26, 2019.

