Our capabilities and experience
Read more about how we help manage complex clinical research across many therapeutic areas worldwide.
Page 4 of 6
We Are Mission Driven
Our team is caring, passionate and working towards the same goal: to address unmet research needs and achieve maximum social impact by supporting the development of life-saving vaccines and medicines. Watch the video to learn why our team thinks being mission-driven makes FHI Clinical a special place to work.
We Partner With Our Sites
Our site managers partner with study sites to ensure their success throughout the length of the project. This can include training and re-training for procedures, processes, protocol, ICH GCP, data management, informed consent and more.
How We Build Research Capacity
We believe that building and sustaining research capacity, particularly in resource-limited, research-naïve areas, will help address public health issues around the globe. Watch the video to learn how our teams support sites to develop sustainable research capacity and produce high-quality study data. In addition, we work with regional institutions to establish networks of research-ready sites that focus on local health and research priorities.
Coronaviruses Fact Sheet
Discovered in the 1960s, coronaviruses (CoV) are a large group of RNA viruses in the family Coronaviridae that cause disease in humans and animal species. Human illness includes respiratory infections that range from the common cold to viral (direct) or bacterial (indirect) pneumonia and are especially serious in infants and at-risk adults. There are seven known coronavirus types/strains.
About the PREVAIL Network
The Partnership for Research on Vaccines and Infectious Diseases in Liberia (PREVAIL) network was established through a partnership between local Liberian and international partners, including the U.S. National Institutes of Health, World Health Organization and FHI Clinical, to provide sustainable research infrastructure to address diseases of public health importance.
Celebrating Our 2-Year Anniversary
We’re proud of our progress and remain committed to and passionate about our mission-driven work globally.
Phase II Trial of a Chikungunya Vaccine in the Caribbean
The study team maintained a 91% retention rate in a study of a Chikungunya virus (CHIKV) vaccine, despite study disruptions due to natural disasters, including Hurricane Maria, and political unrest near the sites in five Caribbean countries in a CHIKV endemic region.
How Can We Streamline the Vaccine Development Process?
To address the urgency of vaccine development during a pandemic, innovative strategies are implemented across study design, regulatory processes, recruitment process and more. In this e-book, we describe some of those innovations and argue for incorporating these into the fabric of clinical trials — to establish a “new normal.”
Site Monitoring & Management in a Clinical Trial of a Spatial Repellent for Vector-Borne Disease Control
The FHI Clinical project team supported site monitoring, site management and project management to assist the University of Notre Dame investigators achieve a level of rigor not typically required for a non-medical product in their double-blinded, randomized-cluster, placebo-controlled clinical trial of a spatial repellent against mosquitoes for malaria prevention.
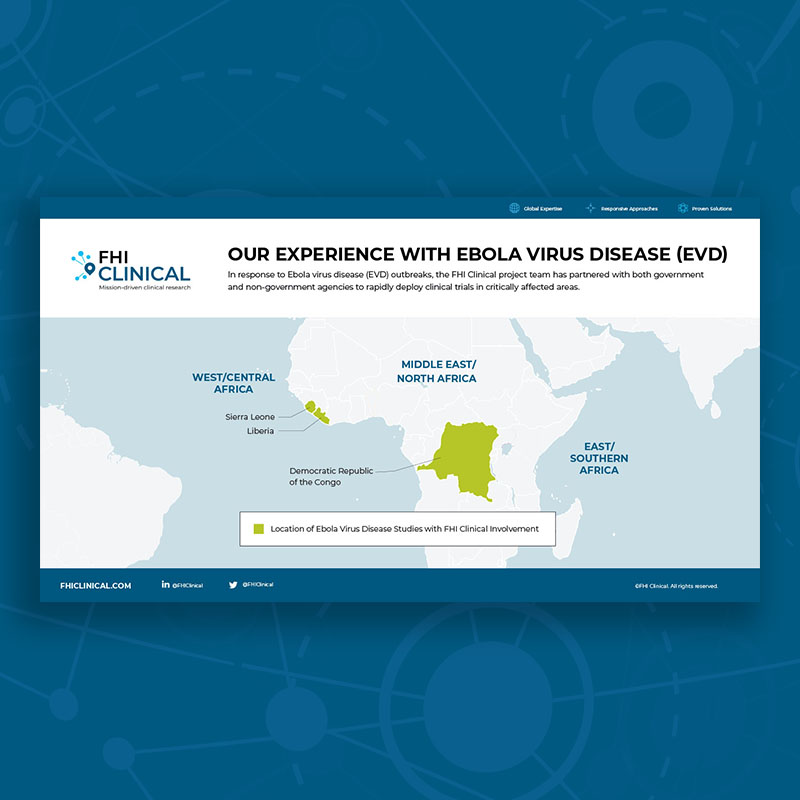
Our Clinical Trial Experience With Ebola Virus Disease
Since 1976, Ebola virus disease outbreaks have primarily occurred in eleven countries: Democratic Republic of the Congo, Gabon, Guinea, Liberia, Mali, Nigeria, Republic of Congo, Sierra Leone, Sudan and Uganda. Although it is generally rare, it is often fatal, with death rates ranging from 25 to 90% in past outbreaks (average case fatality rate, 50%). No treatment or vaccine is currently approved, but there are several promising vaccines and treatments under development.