
Confidently conduct research in
sub-saharan africa
we have a broad geographic footprint
Leverage our unique, localized view using our established resources and research experience in 389 studies across the 31 countries highlighted in blue in the map below.
Our far-reaching, in-country technical, regulatory and cultural knowledge has been developed over our long history of working in sub-Saharan Africa.
We’ve supported first-in-human to phase 4 clinical trials as well as epidemiological, observational and bioequivalence studies for pediatric and adult populations.
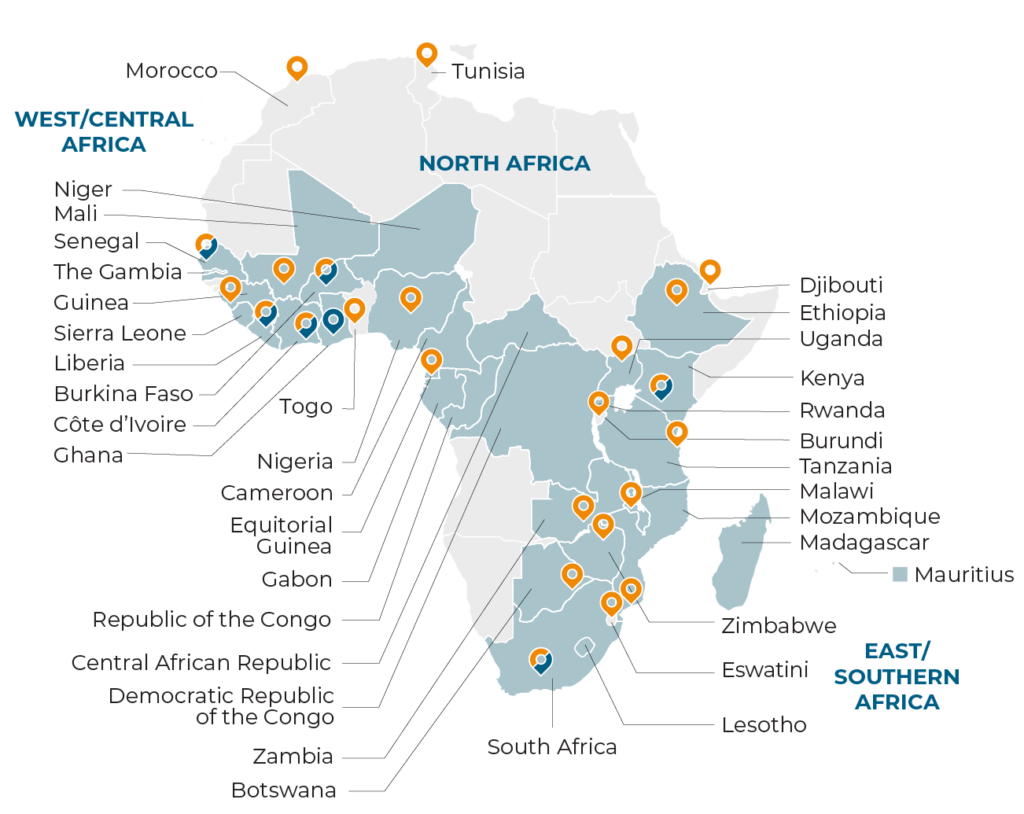
Data current as of July 1, 2023. The boundaries and names on this map do not imply official endorsement or acceptance by FHI Clinical.

FHI Clinical Presence

FHI Clinical & FHI 360

FHI 360 Locations
We are localized
Our localized team has the experience and skills to support your portfolio strategy, determine feasibility, rapidly develop sites and provide end-to-end study support.
Our experience helps guide decisions about the best-fit countries and populations for your target indication and study type while being sensitive to cultural nuances and the specific needs of the local communities.
We have an FHI Clinical SA office in Centurion, South Africa and FHI Clinical offices in Nairobi, Kenya, and Monrovia, Liberia.
WE SUPPORT MANY THERAPEUTIC AREAS
Leverage our previous and ongoing experience conducting studies in sub-Saharan Africa across nearly 20 therapeutic areas in both pediatric and adult populations.
SPECIFIC INFECTIOUS DISEASE EXPERTISE IN SUB-SAHARAN AFRICA
Given the high burden of infectious diseases, neglected tropical diseases and emerging and reemerging diseases in vulnerable populations in this geographic area, we are committed to helping eradicate these diseases through a combination of vaccines, preventive therapies, spatial repellents and treatments. Our experience spans 236 studies across 26 indications.
we are a trusted partner
Participation in research networks
As a trusted network partner, we have unique insights that contribute to protocol development, regulatory oversight, community engagement, liaising with experienced investigators and sites and, when needed, building sustainable research capacity that aligns with the local health and research priorities.
We build sustainable local capacity.
Sites in Africa
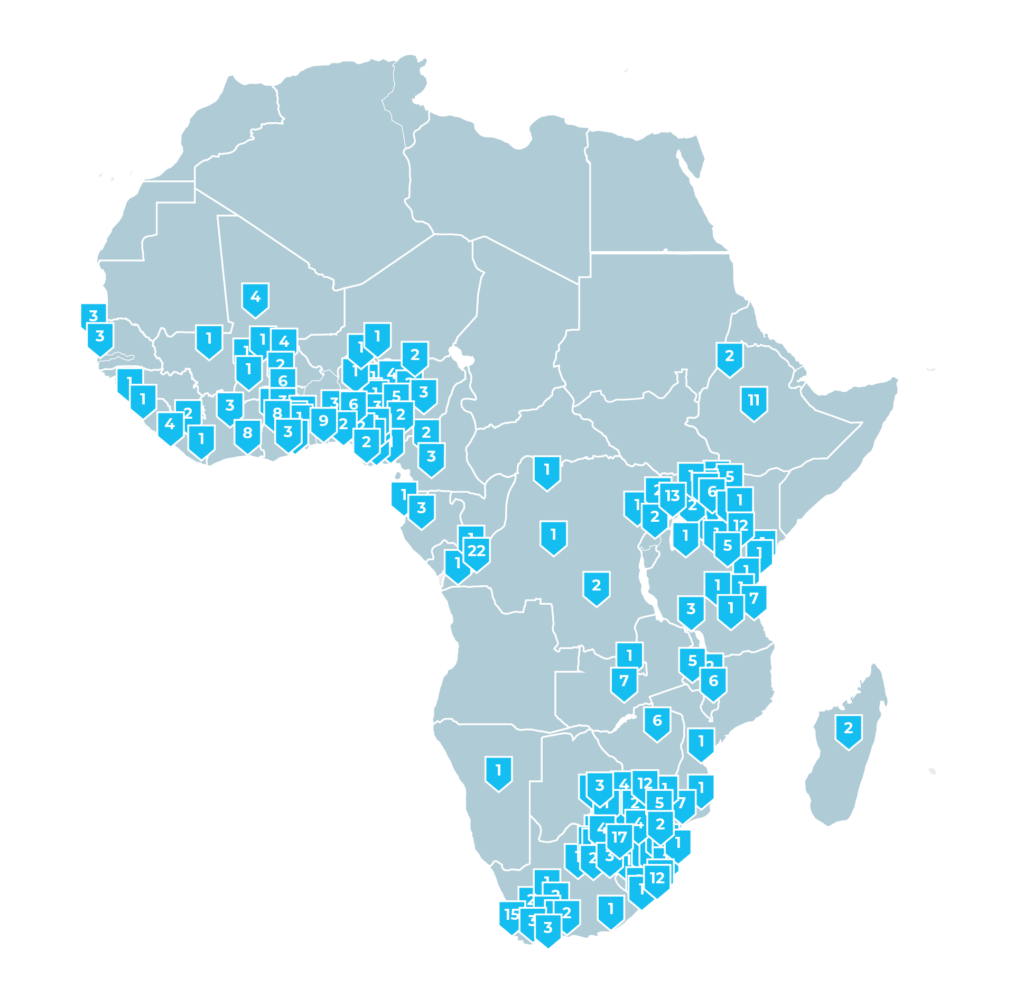
Are you a site interested in being considered for future studies with FHI Clinical? Complete the form, and we will contact you to arrange a formal introduction with the FHI Clinical team for further evaluation of your site.
Data current as of July 1, 2023. The boundaries and names on this map do not imply official endorsement or acceptance by FHI Clinical.












