We’ve previously described the unique diversity in population, pathogenic profile, environment and disease epidemiology as well as the experienced investigators and sites and local investment in growing the research capacity on the African continent. Another key consideration for clinical research is the regulatory environment for postmarketing activities of the investigational products. How difficult and time-consuming is it to have a new product approved for use in African countries?
Inefficient regulatory processes can limit the introduction of new medicines.
In fact, dissonance in regulatory processes and timelines between countries in Africa has been cited by sponsors and CROs as a deterrent to pursuing research in the area, particularly multinational research. Inefficient processes have also been a complaint, with lag times of 4 years to 7 years between first regulatory submission to a well-resourced National Medicine Regulatory Authority (NMRA) and final approval reported for countries in sub-Saharan Africa.10 Historically, each country operated under its own autonomy, and drug and device approvals from country to country have required skillful navigation and local knowledge of the nuances of that regulatory agency.
Frustration with convoluted regulatory processes and a lack of harmonization in regulations between countries are not new to the life sciences industry, regardless of where trials are being conducted. The need to submit multiple dossiers, undergo multiple inspections and pay multiple fees for different countries adds time and expense to marketing approval and has been a reason for some companies to limit the number of countries for submission1 This can limit access to new and life-saving medicines in areas of need, negatively impacting public health. The effect on their populations has motivated governments worldwide to pursue harmonization at regional and global levels. For example, the European Medicines Agency (EMA), established in 1995, and European Commission oversee recommendations for drug, vaccine and device approvals for its 27 member countries.
Africa is no different—governments aiming to improve their population’s health and address priority diseases are working together to enhance and standardize regulatory processes. With the task of bringing together 54 separate countries on the African continent, each with its own NMRA, reaching a common approach to regulatory oversight for investigational products has understandably been an iterative approach.
As we work with our partners to plan and conduct clinical trials, and ultimately marketing of their products, in Africa, we like to highlight the progress that has been made to streamline regulatory processes. Harmonization initiatives are ongoing, and the information here captures the situation at the time of writing. Our teams stay current with recent regulatory developments, so feel free to contact us if you’re interested in evaluating new products in Africa.
Successful regional harmonization has paved the way for expansion across the continent.
Initiation of the African Medicines Regulatory Harmonization (AMRH) initiative in 2009 was a hallmark step in the process of continent-wide harmonization.3 Implemented within the African Union (AU) Pharmaceutical Marketing Plan of Africa (PMPA)14 and supported by a number of international organizations, its objective is to ensure African people have access to essential medical products and devices.
African Medicines Regulatory Harmonization (AMRH)
HISTORY: Founded in 2009
GOAL: Encourage a harmonized approach to the regulation of medicine.
Human and institutional
capacity development
capacity development
Policy alignment
Regional integration and harmonization
PARTNERS
- African Union Commission (AUC)
- Pan-African Parliament (PAP)
- World Health Organization (WHO)
- Bill and Melinda Gates Foundation
- World Bank (WB)
- UK Department for International Development (DFID)
- US Government-PEPFAR
- Global Alliance for Vaccines and Immunization (GAVI)
To encourage a shift from country-focused approaches to collaborative regional regulatory oversight, the AMRH also supported the eight African regional economic communities (RECs) in improving regulatory standards and expediting the registration of essential medicines.
The AU recognizes eight RECs7:
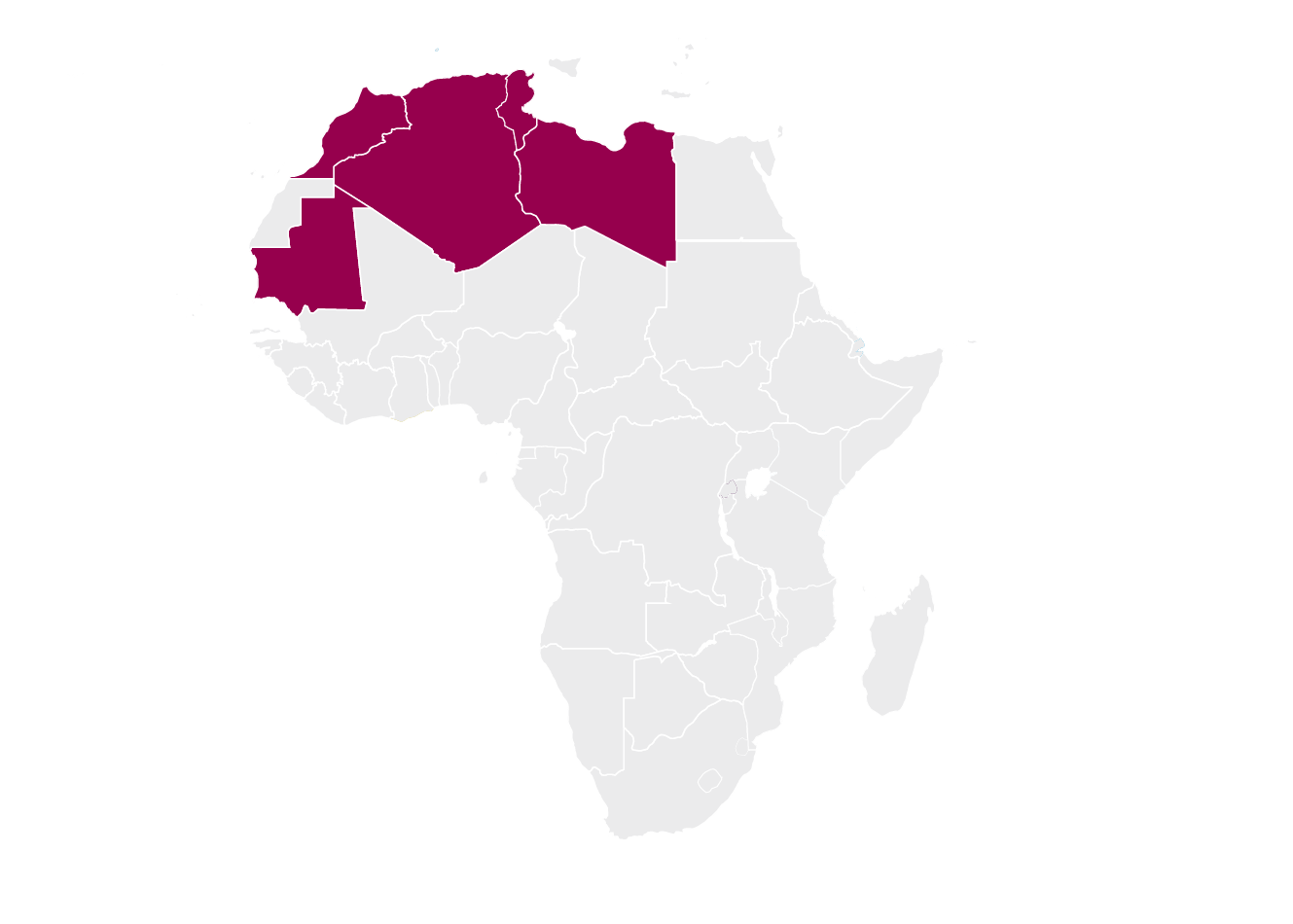
Arab Maghreb Union (AMU)
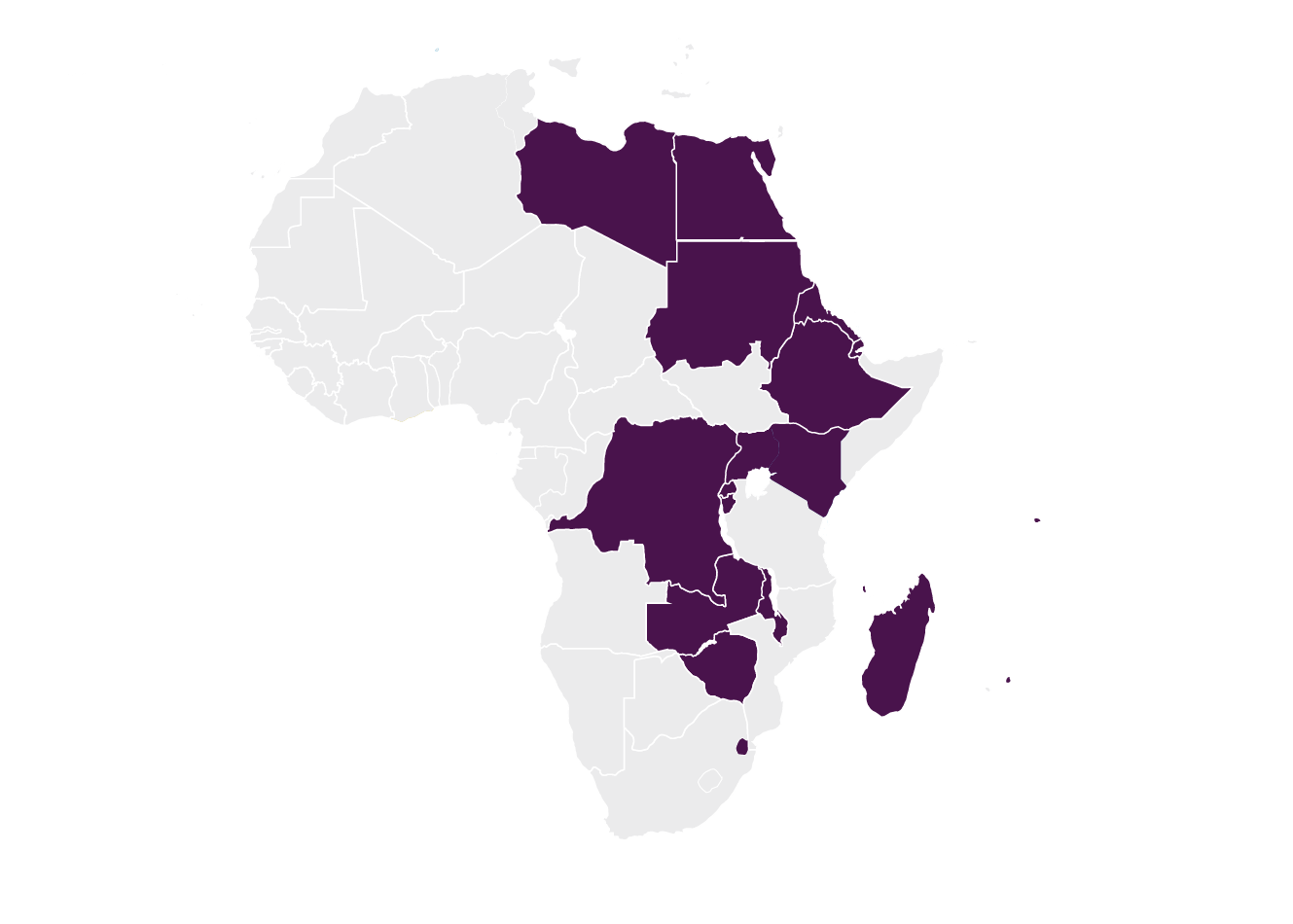
Common Market for Eastern and Southern Africa (COMESA)
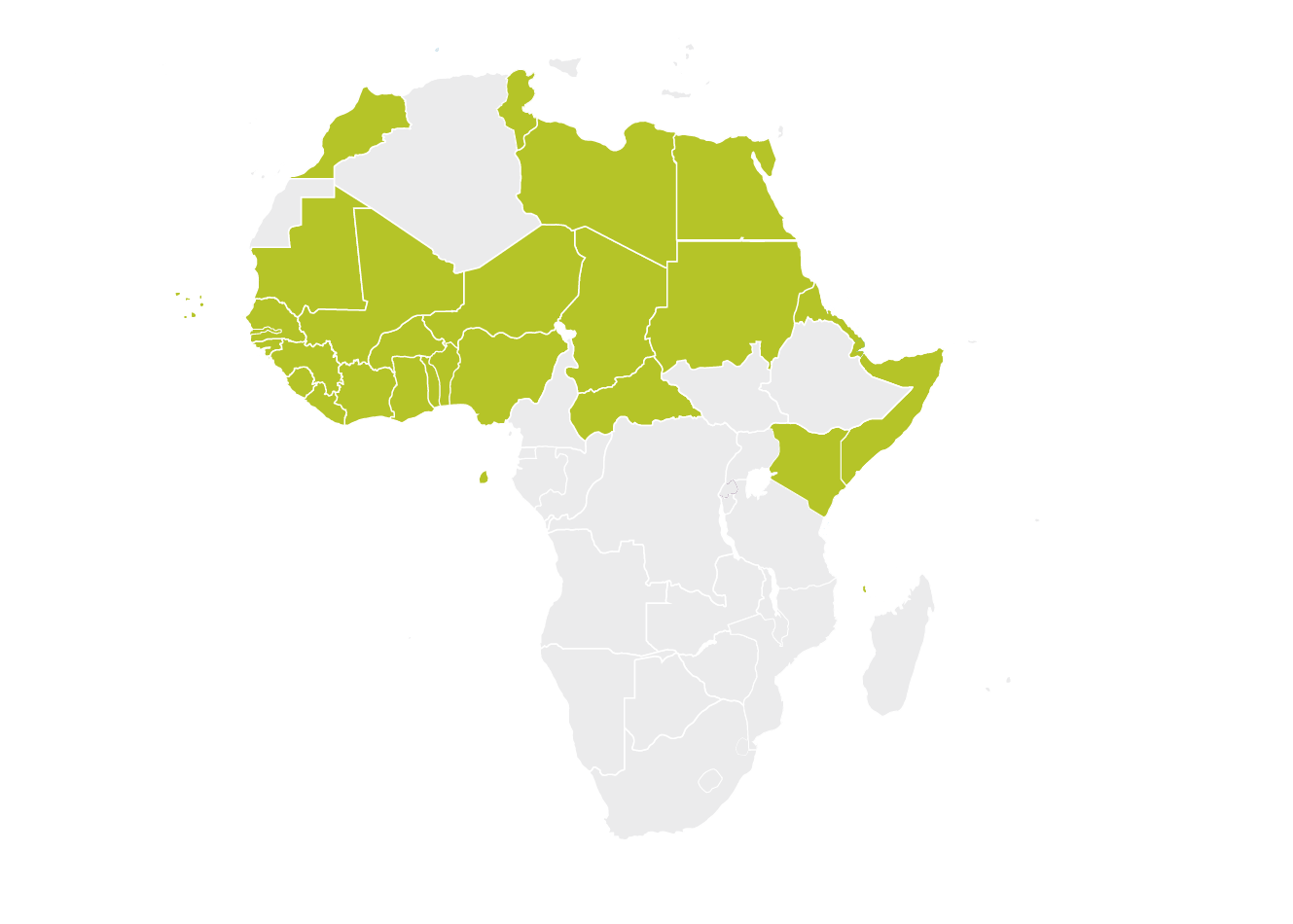
Community of Sahel-Saharan States (CEN-SAD)
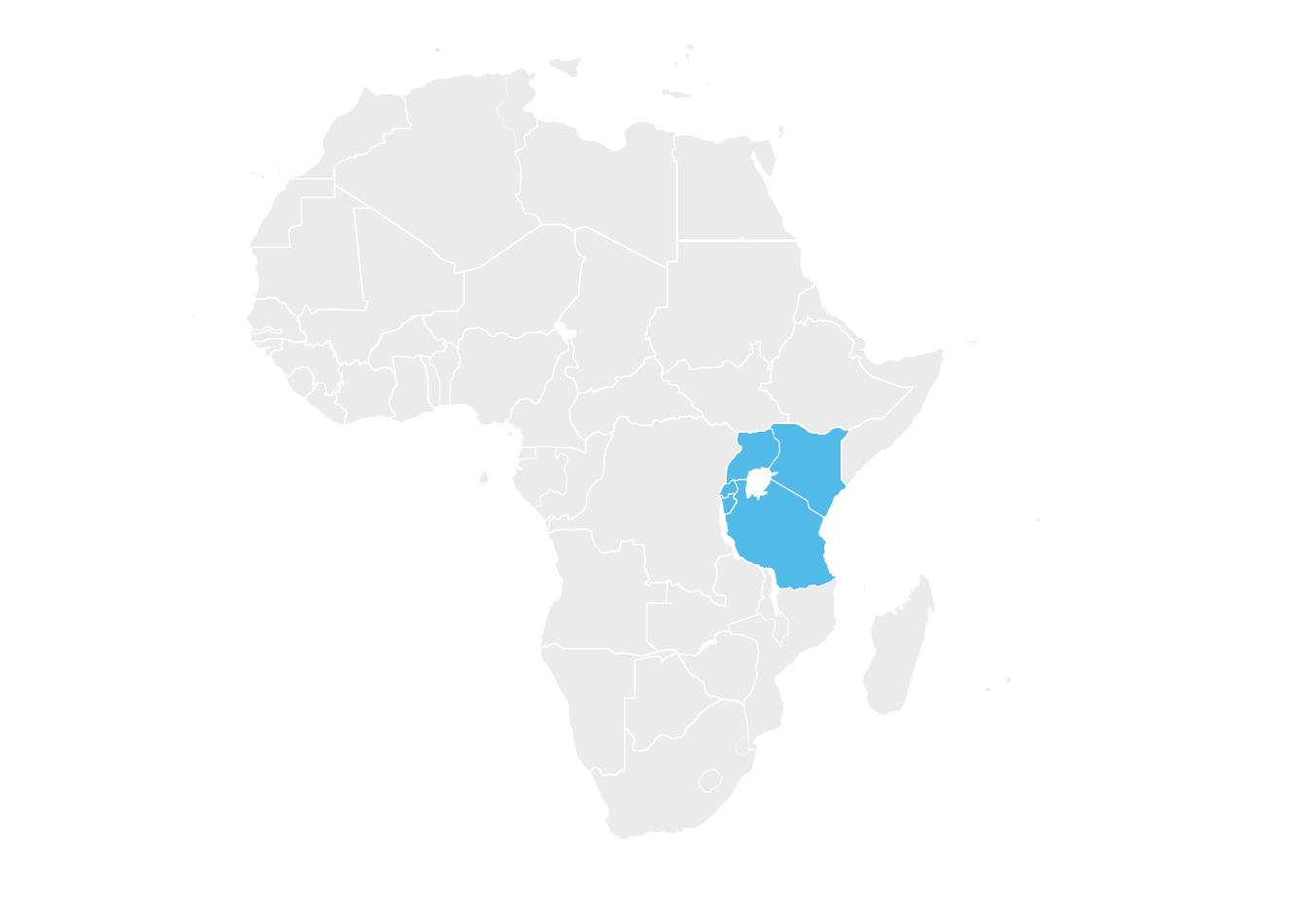
East African Community (EAC)
Economic Community of Central African States (ECCAS)
Economic Community of West African States (ECOWAS)
Intergovernmental Authority on Development (IGAD)
Southern African Development Community (SADC)
The first REC pilot was initiated in the EAC in 2012, and harmonized standards and tools for the registration of medicines in the EAC were implemented in early 2015.8 The first major milestone occurred later in 2015, when Roche was the first company to apply for a single, region-wide joint assessment in the EAC, for bevacizumab and trastuzumab.9 Not approved for any country within the EAC when Roche submitted its application, both oncology treatments were previously approved by the U.S. Food and Drug Administration (FDA) and listed on the World Health Organization’s list of essential treatments. Based on the submission, both drugs were recommended for registration in all EAC countries. This accomplishment demonstrated the feasibility of regional submissions and paved the way for future approvals.
Since then, the EAC has reduced registration times and recommended many important medicines for use in the region.9

Other RECs have followed suit and made strides in standardizing and streamlining registration processes. Through these, the AMRH initiative has helped to build trust, confidence and ownership in the regulation of medical products and health technologies.
Three key accomplishments have resulted from the cross-regional AMRH initiative.
Other key accomplishments related to the AMRH initiative include the development of the AU Model Law on Medical Products Regulation, establishment of the African Union Development Agency-New Partnership for Africa’s Development (AUDA-NEPAD) and the ratification of the African Medicines Agency (AMA) by many countries in Africa.
African Medicines Regulatory Harmonization (AMRH)

AU Model Law
Endorsed by the African Heads of State and Government at the AU Summit in January 2016, the AU Model Law on Medicinal Products Legislation provides a framework for good medicine regulation at a national level and is intended as guidance for AU Member States to address the gaps and inconsistencies in regulatory legislation and enable harmonization.4
It outlines key functions and standards that should be used by each country’s NMRA:
- Registration and authorization of all medical products
- Licensing for manufacturing and distribution of health technologies
- Monitoring and analyzing the quality and safety of health technologies
- Inspections of manufacturing facilities
- Review and authorization of clinical trials
- Establishment of administrative appeals committees
AUDA-NEPAD
In 2018, the NEPAD Planning and Coordination Agency was re-established as the AUDA-NEPAD with additional programs centered around human capital development, science, technology and innovation, and regional integration. It was tasked with integrating research and innovation within the “Africa Health Strategy 2016-2030,”2 which recognizes the interdependency of public health initiatives and equitable access to new medicines.
The Africa Health Strategy was adopted in line with the AU Agenda 2063 and the United Nation Sustainable Development Goals (SDG)5,6 and prioritizes investment in research and innovation to address the challenges across the African continent. To achieve this end, the Health Research and Innovation Strategy for Africa (HRISA) was developed to provide guidance for Member States and prioritize the following:
- Establishing functional national and institutional ethics and regulatory committees that provide timely and efficient review
- Strengthening and harmonizing national and regional regulatory systems
- Adopting a regional approach to strengthen health research oversight
This strategy envisions an Africa where African-led research and innovation drives health and wellbeing.
AMA
Along the same lines of strengthening capabilities within African countries, the AMA was established with the vision of ensuring all Africans have access to quality-assured, safe, efficacious and affordable medical products that meet internationally recognized standards, for priority diseases or conditions. Building on the experiences of the REC MRHs, including those of the EAC and SADC, the AMA:
- Provides a single approach to product review and approvals across the continent based on the AU Model Law
- Improves regulatory harmonization and reliance
- Increases efficiency and effectiveness by pooling expertise and capacities and strengthening existing networks.
Although adopted in February 2019, in order for the AMA to go into effect, 15 countries had to sign and ratify the AMA treaty. In June 2019, Rwanda was the first Member State to sign the treaty,11 and as of October 5, 2021, 15 countries had ratified the treaty, thereby formalizing the institution12 as the second specialized health agency of the AU following the Africa Centers for Disease Control (CDC).
One year after it was ratified, as of October 11, 2022, a total of 23 countries have ratified, 11 countries have signed but not ratified, and 21 countries have yet to sign the treaty.13
With each country that joins the AMA, every other Member State benefits from additional research experience, investigators and resources; their populations gain greater access to affordable, quality medicines; and sponsoring institutions can conduct research within a harmonized framework and a greater population.
It’s time to consider Africa as a clinical trials destination.
Our teams on the ground have witnessed the progress in streamlining regulatory processes firsthand. It is exciting to see areas in which we have been working for decades successfully implementing a common approach to medicines regulation that will provide greater and more affordable access to vaccines, therapies and devices for their populations. We encourage companies that have not considered Africa to be a clinical trials destination previously to reconsider, explore the opportunities on the continent and take advantage of our experience and local knowledge to find the right local partners.
References
- Narsai K, Williams A, Mantel-Teeuwisse AK. Impact of regulatory requirements on medicine registration in African countries – perceptions and experiences of pharmaceutical companies in South Africa. South Med Rev 2012;5(1):31-37. https://pubmed.ncbi.nlm.nih.gov/23093897/
- Health Research and Innovation Strategy for Africa (HRISA): 2018-2030. Africa Union Development Agency. Available at: https://www.nepad.org/publication/health-research-and-innovation-strategy-africa-hrisa-2018-2030
- African Medicines Regulatory Harmonisation (AMRH). African Union Development Agency. Available at: https://www.nepad.org/programme-details/998.
- African Union Model Law for Medical Products Regulation: Increasing access to and delivery of new health technologies for patients in need. United Nations Development Programme. Available at: https://adphealth.org/upload/resource/AU%20Model%20Law.pdf
- Agenda 2063: The Africa We Want. African Union. Available at: https://au.int/en/agenda2063/overview.
- Sustainable Development Goals. United Nations. Available at: https://sdgs.un.org/goals.
- Regional Economic Communities (RECs). African Union. https://au.int/en/organs/recs.
- Ncube BM, Dube A. Ward K. Establishment of the African Medicines Agency: progress, challenges and regulatory readiness. J of Pharm Policy and Pract 2021;14:29. https://doi.org/10.1186/s40545-020-00281-9
- Mashingia JH, Ahonkhai V, Aineplan N, Ambali A, Angole A, Arik M, et al. (2020) Eight years of the East African Community Medicines Regulatory Harmonization initiative: Implementation, progress, and lessons learned. PLoS Med 17(8): e1003134. https://doi.org/10.1371/journal.pmed.1003134
- Ahonkhai V, Martins SF, Portet A, et al. Speeding Access to Vaccines and Medicines in Low- and Middle-Income Countries: A Case for Change and a Framework for Optimized Product Market Authorization. PLoS ONE 2016;11(11): e0166515. https://doi.org/10.1371/journal.pone.0166515
- The Republic of Rwanda Signs the Treaty for the Establishment of the African Medicine Agency (AMA). African Union. June 12, 2019. Available at: https://au.int/en/pressreleases/20190612/republic-rwanda-signs-treaty-establishment-african-medicine-agency-ama
- Pincombe M, Guzman J. A Defining Moment for Medicines Regulation in Africa: The Establishment of the African Medicines Agency. Center for Global Development. February 3, 2022. Available at: https://www.cgdev.org/blog/defining-moment-medicines-regulation-africa-establishment-african-medicines-agency
- Adepoju P. South Africa’s Cabinet Approves African Medicines Agency Treaty. Health Policy Watch: Independent Global Health Reporting. October 11, 2022. Available at: https://healthpolicy-watch.news/south-africa-african-medicines-agency
- Byaruhanga J. The Pharmaceutical Manufacturing Plan for Africa. AUDA-NEPAD. August 24, 2020. Available at: https://www.nepad.org/news/pharmaceutical-manufacturing-plan-africa

